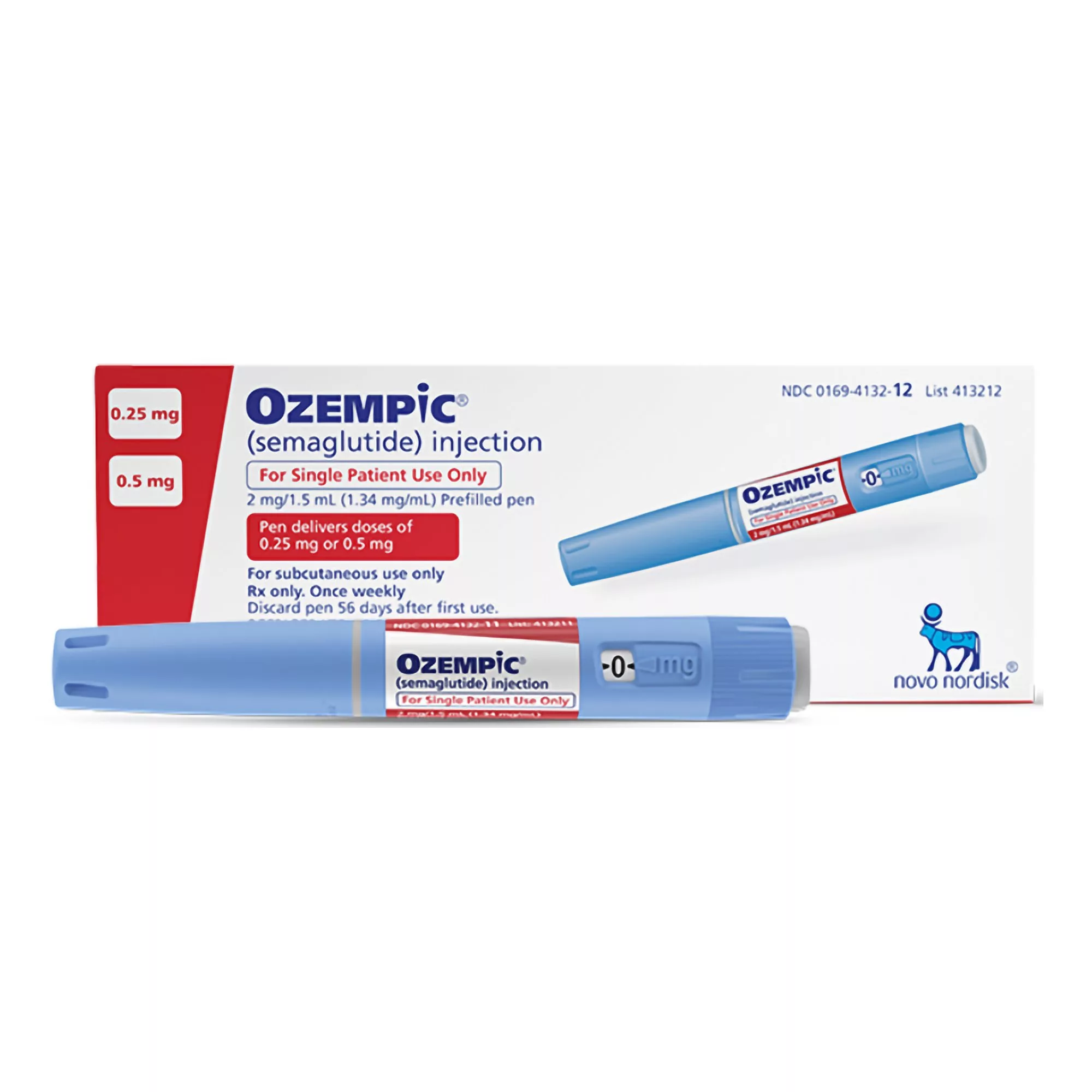
Semaglutide Novo Nordisk – 2ml of Ozempic 0.5mg in 4 doses
$805.00 – $5,880.00
| Active ingredient | Semaglutide |
| Producer | Novo Nordisk |
| Treatment | Diabetes & Weight loss |
| Packaging | Box |
SKU: 18377-1712598169-1-2-1-1
Categories: Buy Semaglutide Online, Super Sale Products
Indications and Limitations of Use
Ozempic® (semaglutide) injection 0.5 mg, 1 mg, or 2 mg is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus and to reduce the risk of major adverse cardiovascular (CV) events (CV death, nonfatal myocardial infarction, or nonfatal stroke) in adults with type 2 diabetes mellitus and established CV disease.
- Ozempic® has not been studied in patients with a history of pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis.
- Ozempic® is not indicated for use in patients with type 1 diabetes mellitus.96
Important Safety Information
Contraindications
- Ozempic® is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with a hypersensitivity reaction to semaglutide or to any of the excipients in Ozempic®. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic®.
Warnings and Precautions
- Risk of Thyroid C-Cell Tumors: Patients should be referred to an endocrinologist for further evaluation if serum calcitonin is measured and found to be elevated or thyroid nodules are noted on physical examination or neck imaging.
- Pancreatitis: Acute and chronic pancreatitis have been reported in clinical studies. Observe patients carefully for signs and symptoms of pancreatitis (persistent severe abdominal pain, sometimes radiating to the back with or without vomiting). If pancreatitis is suspected, discontinue Ozempic® promptly, and if pancreatitis is confirmed, do not restart.
- Diabetic Retinopathy Complications: In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic® (3.0%) compared with placebo (1.8%). The absolute risk increase for diabetic retinopathy complications was larger among patients with a history of diabetic retinopathy at baseline than among patients without a known history of diabetic retinopathy.
Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. The effect of long-term glycemic control with semaglutide on diabetic retinopathy complications has not been studied. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy. - Never Share an Ozempic® Pen Between Patients: Ozempic® pens must never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens.
- Hypoglycemia: Patients receiving Ozempic® in combination with an insulin secretagogue (eg, sulfonylurea) or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
- Acute Kidney Injury: There have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, which may sometimes require hemodialysis, in patients treated with GLP-1 receptor agonists. Some of these events have been reported in patients without known underlying renal disease. A majority of the reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration. Monitor renal function when initiating or escalating doses of Ozempic® in patients reporting severe adverse gastrointestinal reactions.
- Hypersensitivity: Serious hypersensitivity reactions (eg, anaphylaxis, angioedema) have been reported in patients treated with Ozempic®. If hypersensitivity reactions occur, discontinue use of Ozempic®; treat promptly per standard of care, and monitor until signs and symptoms resolve. Use caution in a patient with a history of angioedema or anaphylaxis with another GLP-1 receptor agonist.
- Acute Gallbladder Disease: Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In placebo-controlled trials, cholelithiasis was reported in 1.5% and 0.4% of patients treated with Ozempic® 0.5 mg and 1 mg, respectively, and not reported in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
Adverse Reactions
- The most common adverse reactions, reported in ≥5% of patients treated with Ozempic® are nausea, vomiting, diarrhea, abdominal pain, and constipation.
Drug Interactions
- When initiating Ozempic®, consider reducing the dose of concomitantly administered insulin secretagogue (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia.
- Ozempic® causes a delay of gastric emptying and has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised.
Use in Specific Populations
- There are limited data with semaglutide use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. Discontinue Ozempic® in women at least 2 months before a planned pregnancy due to the long washout period for semaglutide.
3 reviews for Semaglutide Novo Nordisk – 2ml of Ozempic 0.5mg in 4 doses
Add a review Cancel reply
You must be logged in to post a review.
Related products
Sale!
$201.00 – $1,411.00
$600.00 – $7,050.00
$320.00 – $3,000.00
$800.00 – $10,650.00
$538.00 – $2,428.00
Sale!
$159.00 – $1,209.00
Sale!
$114.00 – $864.00
Sale!
$120.00 – $900.00




Smith –
I began taking this medication off-label specifically for weight loss, as I’m not diabetic. I started on August 30, 2020. During the first two weeks, I experienced some mild nausea and dizziness, but it wasn’t too bad. Over the last two weeks, I haven’t had any side effects at all.
My appetite has definitely changed—if I try to eat the way I used to, I end up feeling unwell. I’m currently on a weekly dose of 0.25 mg and following the Weight Watchers program. In just 26 days, I’ve lost 10.5 pounds! My cravings for sweets have pretty much disappeared, and I’m feeling really positive about the progress.
Olivia R –
I was initially hesitant to try Ozempic for weight loss—I needed to lose 30 pounds, but with a busy life and many responsibilities, I couldn’t afford to deal with nausea, diarrhea, or any debilitating side effects. After letting the pen sit in the fridge for weeks, I finally decided to give it a try, and I’m so glad I did.
I’ve now been on the 0.25 mg dose for six weeks, and it’s working well for me—so much so that I don’t feel the need to increase the dosage. Amazingly, I haven’t experienced any side effects at all, not even a headache. The biggest change has been my complete disinterest in food, which feels incredibly freeing.
For the first time in 30 years, I’m not going to bed filled with guilt or making promises to “do better tomorrow.” I don’t crave sugar, and food no longer feels like a reward or a source of comfort. I’m never overly hungry, so I’m able to make calm, healthy choices about what I eat. This has truly been a life-changing experience and has completely shifted the way I view food.
Ethan B –
I love it (not the cost though, I pay for it out of pocket), it’s done wonders for my blood sugar levels. My hbA1c went from 6.6 to 5.8 in three months. Six months in, it’s down to 5.6, and this is with going off my low carb diet. I can actually eat some rice now and it doesn’t spike me over 10 mmol/l. In fact I just had pasta for dinner (very little protein or fats with it) and my blood sugar only went up to about 6.5mmol/l or 117mg/dl. I can drink a Starbucks toffee nut latte and it barely spikes me.
The other bonus is losing weight – it’s a slow but steady loss of about 1kg or 2lbs a month. It’s a function of not having food noise anymore, and my appetite being greatly reduced.
I’m fortunate to have virtually no side effects. I had none at 0.25mg, and occasional nausea in the mornings at 0.5mg and my current dose of 1mg. I do find that if I eat foods that are too rich or oily, I might throw it up a few hours later. It’s only happened 3 or 4 times in the six months I’ve been on Ozempic tnough. And i feel that these are nothing compared to the benefits for me.